
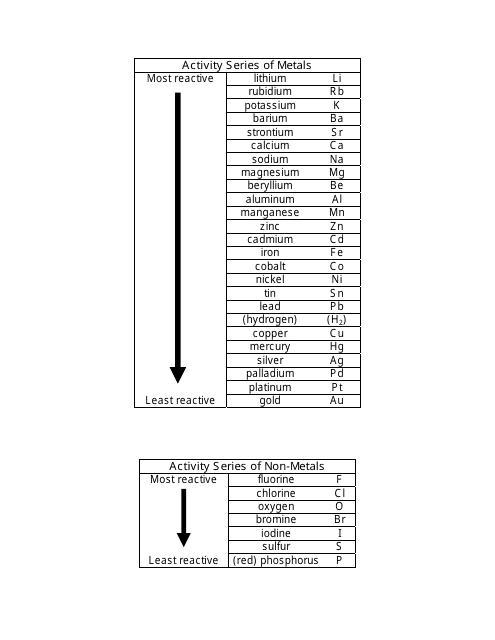
What is the activity series of non metals? How can activity series be used to predict reactions? How is the experiment done to determine the activity series of metals? The mnemonic to remember the reactivity series of metal is : Please send cats, monkeys and zebras in lovely happy cages made of silver, gold & platinum.The reactivity series is the series of metals arranged in decreasing order of their reactivity..For nonmetals, greater activity means a greater ease of gain of electrons, to form negative ions. For metals, greater activity means a greater ease of loss of electrons, to form positive ions. What is meant by the activity of an element?Īn element’s activity is its ability to react. How do you use activity series in chemistry? The series is based on empirical data on the ability of a metal to displace hydrogen gas from water and acid. The reactivity series is also known as the activity series of metals. The reactivity series is an ordering of metals from most reactive to least reactive. What is the difference between reactivity and activity series? Activity series are generally listed in order of decreasing reactivity. Once organized, an activity series helps us to predict if a reaction will occur when a piece of elemental metal is placed in water, an acid solution, or a solution containing the ion of another metal. What is the importance of activity series? Most reactive metals are on top and least reactive metals are on the botton of the list. The reactivity of metals is determined using single-replacement reaction and the positions are determined based on their reactivity. The activity series of metals is organized based on their reactivity with other metals. How is activity series of metals organized? The elements toward the bottom left corner of the periodic table are the metals that are the most active in the sense of being the most reactive. The primary difference between metals is the ease with which they undergo chemical reactions. How do you determine the activity of a metal? The activity series including these elements would be Mg > Zn > H. so zinc is also more active than hydrogen. What is an example of activity series?Īn activity series is a list of substances ranked in order of relative reactivity. It can be used to predict the products in similar reactions involving a different metal. The activity series of metals is an empirical tool used to predict products in displacement reactions and reactivity of metals with water and acids in replacement reactions and ore extraction. What is an activity series and how is it used? The activity series determines the level of reactivity based on how well a certain element can displace hydrogen gas from acidic solutions and water. The activity series is a type of ordering system for elements, which ranks how reactive a certain element is in relation to other elements.

In the reaction with a hydrogen-ion source, the metal is oxidized to a metal ion, and the hydrogen ion is reduced to H2. The activity or electromotive series of metals is a listing of the metals in decreasing order of their reactivity with hydrogen-ion sources such as water and acids. However, copper can be extracted using carbon or hydrogen.24 What is meant by activity of an ion? What is the activity of a metal? Note that zinc and iron can be displaced from their oxides using carbon but not using hydrogen. Here is the reactivity series including carbon and hydrogen: It is useful to place carbon and hydrogen into the reactivity series because these elements can be used to extract metals. When this layer is removed, the observations are more reliable. This is because its protective aluminium oxide layer makes it appear to be less reactive than it really is. Note that aluminium can be difficult to place in the correct position in the reactivity series during these experiments. The quicker the fizzing, the more reactive the metal. The speed at which hydrogen bubbles are produced tells us how reactive a metal is with acid. The tables show how the elements react with water and dilute acids: Element Observations of the way that these elements react with water, acids and steam enable us to put them into this series. For example: P eople S ay L ittle C hildren M ake A Z ebra I ll C onstantly S niffing G iraffes. The reactivity series of metalsĪ good way to remember the order of a reactivity series of metals is to use the first letter of each one to make up a silly sentence. More reactive metals have a greater tendency to lose electrons and form positive ions. In a reactivity series, the most reactive element is placed at the top and the least reactive element at the bottom.


 0 kommentar(er)
0 kommentar(er)
